ADVERTISEMENT
Interventional Coronary Physiology: A 30-Year Overnight Success Story
 This year, the Society for Cardiovascular Angiography and Interventions (SCAI) celebrated the “40 for 40” event; that is, the 40th anniversary of percutaneous coronary intervention and the 40th anniversary year of the founding of the SCAI. On May 11, 2017, I was honored to be the Frank Hildner Annual Lectureship speaker. This lecture is dedicated to Dr. Hildner, one of the founding fellows of the SCAI and the founding editor of the journal Catheterization and Cardiovascular Interventions (Figure 1). The Hildner lecture series, now in its 17th year, brings innovative technology, important new developments, historical insights and healthcare issues relevant to the practice of interventional cardiology to the SCAI membership. Following the lead of my preceding luminaries in the field, I thought it would be informative and hopefully entertaining to synopsize, from a personal viewpoint, the 30-year history of the important developments that led us to use FFR and now iFR. I’ll share the highlights of this lecture with you.
This year, the Society for Cardiovascular Angiography and Interventions (SCAI) celebrated the “40 for 40” event; that is, the 40th anniversary of percutaneous coronary intervention and the 40th anniversary year of the founding of the SCAI. On May 11, 2017, I was honored to be the Frank Hildner Annual Lectureship speaker. This lecture is dedicated to Dr. Hildner, one of the founding fellows of the SCAI and the founding editor of the journal Catheterization and Cardiovascular Interventions (Figure 1). The Hildner lecture series, now in its 17th year, brings innovative technology, important new developments, historical insights and healthcare issues relevant to the practice of interventional cardiology to the SCAI membership. Following the lead of my preceding luminaries in the field, I thought it would be informative and hopefully entertaining to synopsize, from a personal viewpoint, the 30-year history of the important developments that led us to use FFR and now iFR. I’ll share the highlights of this lecture with you.
Frank Hildner, MD, a past president of SCAI (1989-1990), is a superb clinical, teacher, educator, editor, mentor, and leader in the field of cardiac catheterization and subsequently, the specialty of coronary interventions. In 1987, I was a young faculty member at St. Louis University and recent co-director of the J.G. Mudd Cath Lab. I was astonished to be called by Frank to join him on a committee at the Williamsburg SCAI Annual Scientific Sessions. Meeting this group of nationally and internationally recognized superstars in cardiac catheterization was one of a few high points of my career. My life has never been the same since. By joining the SCAI, I have carried on the traditions and teachings within invasive cardiology. I encouraged many young cardiac cath attendings and others to participate in this society for personal and professional development, as well as to gain increased awareness of patient and procedural needs, furthering the education of our colleagues and fellows.
The Timeline of Interventional Coronary Physiology Development
The evolution of interventional coronary physiology (like PCI) is not simple and straightforward (Figure 2). The twists and turns, and the opposing interactions between the different areas, scientists, and regions of the world while developing techniques to measure coronary blood flow and pressure are complex. Beginning in the 1930’s up to about 1970, there were very few techniques for measuring human coronary blood flow. These early methods, such as nitrogen washout, coronary sinus thermodilution and microspheres were difficult, imprecise, and often impractical. In 1974, Dr. K. Lance Gould, undoubtedly a driving spirit of investigation into human coronary physiology, demonstrated in several elegant animal experiments that coronary flow reserve, or hyperemic maximal to basal flow ratio, could be related to stenosis severity, and perhaps overcome the limitations and ambiguity of the coronary angiogram for detection of ischemia.1
By 1977, Dr. Andreas Grüntzig’s coronary angioplasty balloon catheter had changed cardiology history. Most relevant was that it used a small pressure lumen to measure translesional gradients, not only guiding the success of balloon dilation, but setting the stage to study coronary artery disease in awake patients. Around the same time, coronary Doppler studies were performed in patients undergoing coronary artery bypass graft surgery (CABG). In the 1990’s, pressure wires, along with Doppler-tipped angioplasty wires, became available and with those tools, the science of human intracoronary human coronary physiology took off. By 1996, the concept of a pressure-only coronary reserve measurement, fractional flow reserve, had been born and validated. Over the next 15 years, the clinical outcome studies DEFER, FAME 1, and FAME 22-4 made the use of FFR relevant and an important addition to the practice. Further evolution using novel wave intensity analysis led to the development of the resting pressure ratio of the instantaneous wave-free ratio, iFR, presented at the American College of Cardiology (ACC) Scientific Sessions in March 2017, bringing continued excitement and advancement to intracoronary physiology.
Why Use Coronary Physiology?
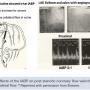
In viewing an angiogram, “I know what a significant lesion is when I see it and I don’t need to measure pressure of flow”, was (and is) commonly heard from many interventionalists. “Of course, (wearing x-ray glasses) I trust my eyes and I know that whatever I see must be true” (Figure 3). However, the angiographic assessment of lumen severity has proven to be a false friend. In 1974, Dr. K. Lance Gould was the first to say this, noting, “The motivation behind clinical coronary physiology is… [driven] by angiographic ambiguity… which intensified the [need for the] noninvasive testing world.” He further stated, in the recent historical review by Johnson et al5 (Figure 4), that “we face today the same quandary as the clinicians faced at the beginning of invasive coronary angiography in 1979. Namely, how should we determine which lesions or lesion to revascularize, and which can be deferred for medical therapy alone”.
 Gould et al’s 1974 study1 was probably the most impactful coronary physiologic study of the modern era. Gould et al measured coronary flow reserve (CFR) in experimental dogs and plotted it against the percent diameter stenosis induced by a micromanometer occluder. CFR began to decline when stenoses were >60% narrowing and resting flow decreased when stenoses were >90% narrowed. Hence, it was thought that coronary physiology could predict anatomy and vice versa. However, in 1993, Marcus et al showed us this was not true in human patients. Compare CFR vs percent stenosis in the 1974 curve of Gould et al to that of Marcus et al in 19886 (Figure 5). In human patients, unlike in the experimental dogs, CFR was poorly related to the angiographic percentage narrowing, because of two factors: 1) the inability of the angiogram to precisely determine the stenosis area and length, and 2) the fact that some patients had microvascular disease and an impaired coronary flow reserve, even with normal coronary arteries.
Gould et al’s 1974 study1 was probably the most impactful coronary physiologic study of the modern era. Gould et al measured coronary flow reserve (CFR) in experimental dogs and plotted it against the percent diameter stenosis induced by a micromanometer occluder. CFR began to decline when stenoses were >60% narrowing and resting flow decreased when stenoses were >90% narrowed. Hence, it was thought that coronary physiology could predict anatomy and vice versa. However, in 1993, Marcus et al showed us this was not true in human patients. Compare CFR vs percent stenosis in the 1974 curve of Gould et al to that of Marcus et al in 19886 (Figure 5). In human patients, unlike in the experimental dogs, CFR was poorly related to the angiographic percentage narrowing, because of two factors: 1) the inability of the angiogram to precisely determine the stenosis area and length, and 2) the fact that some patients had microvascular disease and an impaired coronary flow reserve, even with normal coronary arteries.
 This conundrum caused the field of human coronary physiology to stall, until on July 12, 1979, Dr. Andreas Grüntzig, the first interventional coronary physiologist, reported the first “nonoperative dilatation of the coronary artery stenosis”; that is, percutaneous coronary balloon angioplasty, in the New England Journal of Medicine7 (Figure 6A). This paper showed the method and results of the dilating Grüntzig catheter (DG20x30) inflating in a stenosis. The measurement of the pressure gradient before and after the balloon inflation and deflation was used to guide the procedure, and overcome the very poor angiographic image quality at that time. Pressure measurements during percutaneous transluminal coronary angioplasty (PTCA) were questioned, because of the technical difficulty of measuring pressure through a large 4.5 French (F) catheter with a small, fluid-filled pressure lumen (Figure 6B). Over the next few years, coronary angiography improved dramatically and Dr. Jeff Hartzler, one of the world’s leading interventionalists at that time, did not believe we needed pressure to do angioplasty. The use of pressure measurements returned with a vengeance a decade later.
This conundrum caused the field of human coronary physiology to stall, until on July 12, 1979, Dr. Andreas Grüntzig, the first interventional coronary physiologist, reported the first “nonoperative dilatation of the coronary artery stenosis”; that is, percutaneous coronary balloon angioplasty, in the New England Journal of Medicine7 (Figure 6A). This paper showed the method and results of the dilating Grüntzig catheter (DG20x30) inflating in a stenosis. The measurement of the pressure gradient before and after the balloon inflation and deflation was used to guide the procedure, and overcome the very poor angiographic image quality at that time. Pressure measurements during percutaneous transluminal coronary angioplasty (PTCA) were questioned, because of the technical difficulty of measuring pressure through a large 4.5 French (F) catheter with a small, fluid-filled pressure lumen (Figure 6B). Over the next few years, coronary angiography improved dramatically and Dr. Jeff Hartzler, one of the world’s leading interventionalists at that time, did not believe we needed pressure to do angioplasty. The use of pressure measurements returned with a vengeance a decade later.
The Doppler Flow Wire
 In 1990, I equipped a Judkins 8F catheter with a Doppler crystal at the tip8 (Figure 7A). I thought that measuring left main coronary blood flow velocity with this catheter would be revolutionary for pharmacologic and other interventional studies in the human coronary circulation. It was fortuitous that the same year, Dr. Meno Nassi, CEO of Cardiometrics, Inc., asked if I thought a Doppler-tipped angioplasty guidewire would have any value in the cath lab and PTCA procedures. After passing the flow wire through my Judkins Doppler catheter and realizing I could measure flow everywhere in the coronary tree, I thought, I’ve just obsoleted myself. I said to Meno, “I think so, but let me just test it out a few more times.” I was thrilled. Measuring intracoronary flow velocity opened new doors. Within the next two years, we produced a symposium in the American Journal of Cardiology, entitled “The clinical application of intracoronary Doppler flow velocity in patients: understanding flow beyond the coronary stenosis” (Kern and Anderson on May 20, 1993).9 This symposium detailed the characteristics of coronary flow velocity and its numerous applications (Figure 7B). Coronary flow reserve could now be acquired and measured, in any vessel, in any setting, in any patient in the cath lab.
In 1990, I equipped a Judkins 8F catheter with a Doppler crystal at the tip8 (Figure 7A). I thought that measuring left main coronary blood flow velocity with this catheter would be revolutionary for pharmacologic and other interventional studies in the human coronary circulation. It was fortuitous that the same year, Dr. Meno Nassi, CEO of Cardiometrics, Inc., asked if I thought a Doppler-tipped angioplasty guidewire would have any value in the cath lab and PTCA procedures. After passing the flow wire through my Judkins Doppler catheter and realizing I could measure flow everywhere in the coronary tree, I thought, I’ve just obsoleted myself. I said to Meno, “I think so, but let me just test it out a few more times.” I was thrilled. Measuring intracoronary flow velocity opened new doors. Within the next two years, we produced a symposium in the American Journal of Cardiology, entitled “The clinical application of intracoronary Doppler flow velocity in patients: understanding flow beyond the coronary stenosis” (Kern and Anderson on May 20, 1993).9 This symposium detailed the characteristics of coronary flow velocity and its numerous applications (Figure 7B). Coronary flow reserve could now be acquired and measured, in any vessel, in any setting, in any patient in the cath lab.
 Several landmark studies using intracoronary Doppler were produced from our lab. These included what balloon angioplasty and stenting did to coronary flow reserve.10 It was remarkable to us that PTCA was so poor at increasing flow (CFR increasing from 1.4 before to about 1.7 after balloon angioplasty) and that stenting was so much better, increasing CFR to about 2.5 after stenting. But, equally important was the fact that CFR increased in only 80% of patients. We attributed the failure to increase CFR in the remaining 20% to intrinsic microvascular disease or induced microvascular impairment due to micro emboli of plaque manipulations.
Several landmark studies using intracoronary Doppler were produced from our lab. These included what balloon angioplasty and stenting did to coronary flow reserve.10 It was remarkable to us that PTCA was so poor at increasing flow (CFR increasing from 1.4 before to about 1.7 after balloon angioplasty) and that stenting was so much better, increasing CFR to about 2.5 after stenting. But, equally important was the fact that CFR increased in only 80% of patients. We attributed the failure to increase CFR in the remaining 20% to intrinsic microvascular disease or induced microvascular impairment due to micro emboli of plaque manipulations.
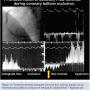 In one of our most unique observations in 1993, during PTCA with a Doppler wire distal to the balloon occlusion, the normal antegrade flow, seen as a positive Doppler signal, suddenly became reversed in some patients.11 While seeing this phenomenon for the first time (Figure 7C), a visiting faculty candidate, Dr. Thomas Donahue, now at Yale, asked me, “Why is the signal reversed?”
In one of our most unique observations in 1993, during PTCA with a Doppler wire distal to the balloon occlusion, the normal antegrade flow, seen as a positive Doppler signal, suddenly became reversed in some patients.11 While seeing this phenomenon for the first time (Figure 7C), a visiting faculty candidate, Dr. Thomas Donahue, now at Yale, asked me, “Why is the signal reversed?”
“Collateral flow, I think…”
“How many times have you seen this?”
“Including this one?…Once!”
We subsequently learned that collateral flow was indeed the cause of this signal inversion, and we could measure the intensity and phasic nature of coronary collateral flow in both chronic and acute conditions. This remarkable demonstration opened up the field for the study of collateral flow and enlarged the universe for studies of human ischemia in our patients undergoing balloon angioplasty.
 Regarding the effects of an intra-aortic balloon pump (IABP), we found that Doppler flow increased flow across mild and moderate stenoses, but had minimal effect to increase flow across severe stenoses, and that in some patients, collateral flow could be increased by intra-aortic balloon pumping; again, a remarkable method to verify proposed and speculative mechanisms of IABP action12 (Figure 8).
Regarding the effects of an intra-aortic balloon pump (IABP), we found that Doppler flow increased flow across mild and moderate stenoses, but had minimal effect to increase flow across severe stenoses, and that in some patients, collateral flow could be increased by intra-aortic balloon pumping; again, a remarkable method to verify proposed and speculative mechanisms of IABP action12 (Figure 8).
Continuous Doppler flow was useful for endothelial function testing13 and Doppler flow velocity patterns were used to examine myocardial viability after acute infarction.14 These studies helped move interventional cardiology forward with a better understanding of myocardial disease and device function.
The Angioplasty Pressure Wire
 At the same time in 1993, while we were working on studies of coronary Doppler, Nico Pijls from Eindhoven, the Netherlands, and Bernard De Bruyne in Aalst, Belgium, were the first to begin using a .014-inch hollow sensor angioplasty guide wire to measure pressure across a stenosis (Figure 9). By 1994, Radi Medical Systems, out of Uppsala, Sweden, had produced a first solid state pressure wire. With the invention of these devices, the evolution of the pressure-only flow reserve theory could be tested, which culminated in the derivation and application of the fractional flow reserve concept.15,16 Pijls and DeBruyne were the first to publish on the fractional flow reserve of the myocardium. FFR is the percent of stenotic flow to normal flow across a stenosis. The flow ratio is computed from the distal/aortic pressure measurements. One important aspect in the development of the FFR was the incorporation of venous pressure to account for collateral flow, making the validation successful in both the animal lab as well as in clinical outcome studies.
At the same time in 1993, while we were working on studies of coronary Doppler, Nico Pijls from Eindhoven, the Netherlands, and Bernard De Bruyne in Aalst, Belgium, were the first to begin using a .014-inch hollow sensor angioplasty guide wire to measure pressure across a stenosis (Figure 9). By 1994, Radi Medical Systems, out of Uppsala, Sweden, had produced a first solid state pressure wire. With the invention of these devices, the evolution of the pressure-only flow reserve theory could be tested, which culminated in the derivation and application of the fractional flow reserve concept.15,16 Pijls and DeBruyne were the first to publish on the fractional flow reserve of the myocardium. FFR is the percent of stenotic flow to normal flow across a stenosis. The flow ratio is computed from the distal/aortic pressure measurements. One important aspect in the development of the FFR was the incorporation of venous pressure to account for collateral flow, making the validation successful in both the animal lab as well as in clinical outcome studies.
 By 1994, live case demonstrations of intracoronary physiologic measurements — FFR and CFR by Doppler — were being performed in the United States and Europe. I was fortunate to be part of the first course, in Eindhoven in January 1994, with 90 European interventional cardiologists attending (Figure 10). In one of our first patients, across a 60% right coronary stenosis, Dr. Bernard De Bruyne and I showed that there was a normal FFR and normal coronary flow velocity reserve (CFVR) — that is, the atypical chest pain was not due to coronary artery obstruction. Dr. Pijls then asked Dr. Patrick Serruys, “What to do next?”
By 1994, live case demonstrations of intracoronary physiologic measurements — FFR and CFR by Doppler — were being performed in the United States and Europe. I was fortunate to be part of the first course, in Eindhoven in January 1994, with 90 European interventional cardiologists attending (Figure 10). In one of our first patients, across a 60% right coronary stenosis, Dr. Bernard De Bruyne and I showed that there was a normal FFR and normal coronary flow velocity reserve (CFVR) — that is, the atypical chest pain was not due to coronary artery obstruction. Dr. Pijls then asked Dr. Patrick Serruys, “What to do next?”
He responded, “Stent it.”
“Why, since flow is normal?” asked Dr. Pijls.
The response: “Well… it looks bad!”
Nico, Bernard, and I realized the long road ahead before intracoronary physiology would have an impact on the behavior of the average interventionalist. Nonetheless, we knew that we had to stick with what we believe was right for the patient.
Coronary physiology teaching continued through annual courses. I recall a debate at the European Heart House in 1996 between FFR and CFVR. After the presentations, with Nico presenting on FFR and me on CFR, Nico asked me, “What did you think?”
I responded, “Well, Nico, you have got me beat. Doppler flow does not work very well for lesion assessment. But those FFR equations, they’re killing me!”
“What do you mean?”
I said, “It’s too much math for interventionists. If it were me, I would just show the adenosine syringe, guidewire, and pressure tracing…and then we can really sell it.”
And that is exactly what happened. That year, the validation of FFR, published in the New England Journal of Medicine in 1996 by Nico, Bernard, and others17, demonstrated a strong relationship to an FFR value of 0.75 with the sensitivity of 88%, specificity of 100%, positive predictive value of 100%, negative predictive value of 88%, and accuracy of 93%.
 The validation and extensive single-center clinical applications of FFR led to multicenter trials over the next 15 years. The most famous studies were the DEFER trial, and the FAME 1 and FAME 2 trials.2-4 The DEFER trial asked whether it is safe to defer lesions by FFR; the answer was “yes”. The FAME 1 trial asked whether it was better to guide stenting based on ischemia and FFR rather than angiography alone, and the answer, again, was “yes”. Lastly, the FAME 2 trial, published in 2014 in the New England Journal of Medicine4, asked whether it is better for patients who are ischemic with positive FFRs to be treated with revascularization rather than optimal medical therapy alone, and the answer was, again, yes for percutaneous coronary intervention (Figure 11).
The validation and extensive single-center clinical applications of FFR led to multicenter trials over the next 15 years. The most famous studies were the DEFER trial, and the FAME 1 and FAME 2 trials.2-4 The DEFER trial asked whether it is safe to defer lesions by FFR; the answer was “yes”. The FAME 1 trial asked whether it was better to guide stenting based on ischemia and FFR rather than angiography alone, and the answer, again, was “yes”. Lastly, the FAME 2 trial, published in 2014 in the New England Journal of Medicine4, asked whether it is better for patients who are ischemic with positive FFRs to be treated with revascularization rather than optimal medical therapy alone, and the answer was, again, yes for percutaneous coronary intervention (Figure 11).
 These remarkable studies led to a dramatic increase in the number of publications, with wide-ranging explorations of intracoronary physiology, covering single-vessel, multivessel, and diffuse disease, prior myocardial infarctions (MIs), bifurcations, left mains, jailed side branches, non-ST-elevation MIs, myocardial bridges, in-stent restenosis, bypass grafts, and transcatheter aortic valve replacement (TAVR). Between 2010 and 2015, the number of published papers increased from 50 to 300. Dr. William Fearon, a colleague from Stanford University, has been highly instrumental in completing the FAME 1 and 2 studies. Despite the fact that I had tried to hire him at least five times from 1993 to 2000, and again three more times from the year 2013 to 2016, Bill is now a world-renowned interventionalist best known for his work in the FAME studies.
These remarkable studies led to a dramatic increase in the number of publications, with wide-ranging explorations of intracoronary physiology, covering single-vessel, multivessel, and diffuse disease, prior myocardial infarctions (MIs), bifurcations, left mains, jailed side branches, non-ST-elevation MIs, myocardial bridges, in-stent restenosis, bypass grafts, and transcatheter aortic valve replacement (TAVR). Between 2010 and 2015, the number of published papers increased from 50 to 300. Dr. William Fearon, a colleague from Stanford University, has been highly instrumental in completing the FAME 1 and 2 studies. Despite the fact that I had tried to hire him at least five times from 1993 to 2000, and again three more times from the year 2013 to 2016, Bill is now a world-renowned interventionalist best known for his work in the FAME studies.
Industry Partners and Sensor Wire Technology
 Along with good data, the need for better equipment brings industry partners to the field. There are now five companies making pressure wires and a micro pressure catheter for use in lesion assessment (Figure 12). This industry attention speaks strongly of the importance of FFR and coronary physiology in our daily practice. FFR became incorporated into the SCAI/ACC/American Heart Association guidelines as a Class 2A in the U.S. guidelines and Class 1A in European Society of Cardiology (ESC) guidelines.
Along with good data, the need for better equipment brings industry partners to the field. There are now five companies making pressure wires and a micro pressure catheter for use in lesion assessment (Figure 12). This industry attention speaks strongly of the importance of FFR and coronary physiology in our daily practice. FFR became incorporated into the SCAI/ACC/American Heart Association guidelines as a Class 2A in the U.S. guidelines and Class 1A in European Society of Cardiology (ESC) guidelines.
What’s iFR?
 3In 2012, a number of my colleagues, leaders in the field, and I were gathered at the commemoration of the FAME studies at TCT. The question was asked, “Can a resting Pd/Pa or iFR really work?” At that time, the answer was universally, “Not likely.” (Actual comments from my colleagues are paraphrased for the photograph, Figure 13A). Despite this dark view, iFR studies continued and randomized comparisons to FFR were undertaken. On March 18, 2017, the iFR randomized multicenter trials — the new kids on the block — were presented by Matthias Götberg and Justin Davies at the late-breaking trials session at the ACC Scientific Sessions (Figure 13B). DEFINE-FLAIR is an international, multicenter trial, and the SWEDEHEART study took place in Denmark, Iceland, and Sweden, randomizing iFR and FFR to treat or no-treat decision points.18,19 These studies, summarized in the May 2017 Clinical Editor’s page in Cath Lab Digest, demonstrated that iFR was non-inferior to FFR in both the DEFINE-FLAIR and SWEDEHEART studies, with only minor differences between them (Figure 13C). In summary, because these studies used resting measurements only, compared to FFR, there were no adenosine-related symptoms, since no adenosine is given. iFR had shorter procedure times by 4.5 minutes, again, because no adenosine was given. The main results demonstrated non-inferior outcomes compared to FFR in low-risk patients. Lastly, because iFR measurements were more frequently nonischemic values, there were fewer stents and CABGs performed in the iFR vs the FFR group. However, we should recall that FFR had and still has better long-term outcomes, better ischemic testing validation, and that the standard against which iFR was compared is FFR. The bottom line for the iFR/FFR debate is that more use of either technique would produce better outcomes (Figure 14).
3In 2012, a number of my colleagues, leaders in the field, and I were gathered at the commemoration of the FAME studies at TCT. The question was asked, “Can a resting Pd/Pa or iFR really work?” At that time, the answer was universally, “Not likely.” (Actual comments from my colleagues are paraphrased for the photograph, Figure 13A). Despite this dark view, iFR studies continued and randomized comparisons to FFR were undertaken. On March 18, 2017, the iFR randomized multicenter trials — the new kids on the block — were presented by Matthias Götberg and Justin Davies at the late-breaking trials session at the ACC Scientific Sessions (Figure 13B). DEFINE-FLAIR is an international, multicenter trial, and the SWEDEHEART study took place in Denmark, Iceland, and Sweden, randomizing iFR and FFR to treat or no-treat decision points.18,19 These studies, summarized in the May 2017 Clinical Editor’s page in Cath Lab Digest, demonstrated that iFR was non-inferior to FFR in both the DEFINE-FLAIR and SWEDEHEART studies, with only minor differences between them (Figure 13C). In summary, because these studies used resting measurements only, compared to FFR, there were no adenosine-related symptoms, since no adenosine is given. iFR had shorter procedure times by 4.5 minutes, again, because no adenosine was given. The main results demonstrated non-inferior outcomes compared to FFR in low-risk patients. Lastly, because iFR measurements were more frequently nonischemic values, there were fewer stents and CABGs performed in the iFR vs the FFR group. However, we should recall that FFR had and still has better long-term outcomes, better ischemic testing validation, and that the standard against which iFR was compared is FFR. The bottom line for the iFR/FFR debate is that more use of either technique would produce better outcomes (Figure 14).
The Future
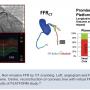 The future of coronary physiology over the next decade is the validation and application of noninvasive imaging-based FFR such as FFRCT (Figure 15). Using 3D reconstruction of the arterial imaging scan and applying computational fluid dynamics, we can generate a virtual FFR. FFRCT has an 80% correspondence to invasive FFR, and data from the PROMISE and PLATFORM studies20,21 showed the ability to reduce procedures by excluding normal angiograms. FFRCT will likely lead the way for further future applications, surpassing our routine stress test approach and focusing the direction of those patients coming to cath lab for PCI rather than merely a diagnostic study. My personal bottom line for the 30-year overnight success story is that this is the best time ever to be involved with coronary physiology and interventional cardiology.
The future of coronary physiology over the next decade is the validation and application of noninvasive imaging-based FFR such as FFRCT (Figure 15). Using 3D reconstruction of the arterial imaging scan and applying computational fluid dynamics, we can generate a virtual FFR. FFRCT has an 80% correspondence to invasive FFR, and data from the PROMISE and PLATFORM studies20,21 showed the ability to reduce procedures by excluding normal angiograms. FFRCT will likely lead the way for further future applications, surpassing our routine stress test approach and focusing the direction of those patients coming to cath lab for PCI rather than merely a diagnostic study. My personal bottom line for the 30-year overnight success story is that this is the best time ever to be involved with coronary physiology and interventional cardiology.
References
- Gould KL, Lipscomb K, Hamilton GW. Physiologic basis for assessing critical coronary stenosis. Instantaneous flow response and regional distribution during coronary hyperemia as measures of coronary flow reserve. Am J Cardiol. 1974 Jan; 33(1): 87-94.
- Zimmermann FM, Ferrara A, Johnson NP, van Nunen LX, Escaned J, Albertsson P, et al. Deferral vs. performance of percutaneous coronary intervention of functionally non-significant coronary stenosis: 15-year follow-up of the DEFER trial. Eur Heart J. 2015 Dec 1;36(45):3182-3188. doi: 10.1093/eurheartj/ehv452.
- Tonino PA, De Bruyne B, Pijls NH, et al.; FAME Study Investigators. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009; 360: 213-224.
- De Bruyne B, Pijls NH, Kalesan B, et al.; FAME 2 Trial Investigators. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012; 367: 991–1001.
- Johnson, NP, Kirkeeide RL, Gould KL. History and development of coronary flow reserve and fractional flow reserve for clinical applications. Intervent Cardiol Clin. 2015 Oct; 4(4): 397-410.
- Wilson RF, White CW. Does coronary artery bypass surgery restore normal maximal coronary flow reserve? The effect of diffuse atherosclerosis and focal obstructive lesions. Circulation. 1987; 76(3): 563-571.
- Grüntzig AR, Senning A, Siegenthaler WE. Nonoperative dilatation of coronary-artery stenosis: percutaneous transluminal coronary angioplasty. N Engl J Med. 1979; 301: 61-68.
- Kern MJ. A simplified method to measure coronary blood flow velocity in patients: validation and application of a Judkins-style Doppler-tipped angiographic catheter. Am Heart J. 1990; 120: 1202-1212.
- Kern MJ, Anderson HV (eds). A symposium: the clinical applications of the intracoronary Doppler guidewire flow velocity in patients: understanding blood flow beyond the coronary stenosis. Am J Cardiol. 1993; 71: 1D-86D.
- Kern MJ, Dupouy P, Drury JH, Aguirre FV, Aptecar E, Bach RG, et al. Role of coronary artery lumen enlargement in improving coronary blood flow after balloon angioplasty and stenting: a combined intravascular ultrasound Doppler flow and imaging study. J Am Coll Cardiol. 1997; 29: 1520-1527.
- Kern MJ, Donohue TJ, Bach RG, Aguirre FV, Caracciolo EA, Ofili EO. Quantitating coronary collateral flow velocity in patients during coronary angioplasty using a Doppler guidewire. Am J Cardiol. 1993; 71: 34D-40D.
- Flynn MS, Kern MJ, Donohue TJ, Aguirre FV, Bach RG, Caracciolo EA. Alterations of coronary collateral blood flow velocity during intra-aortic balloon pumping in patients. Am J Cardiol. 1993; 71: 1451-1454.
- Egashira K, Inou T, Yamada A, Hirooka Y, Marouka Y, Takeshita A. Impaired coronary blood flow response to acetylcholine in patients with coronary risk factors and proximal atherosclerotic lesions. J Clin Invest. 1993; 91: 91-97.
- Kawamoto T, Yoshida K, Akasaka T, Hozumi T, Takagi T, Kaji S, Ueda Y. Can coronary blood flow velocity pattern after primary percutaneous transluminal coronary angioplasty [correction of angiography] predict recovery of regional left ventricular function in patients with acute myocardial infarction? Circulation. 1999 Jul 27; 100(4): 339-345.
- Pijls NH, van Son JA, Kirkeeide RL, De Bruyne B, Gould KL. Experimental basis of determining maximum coronary, myocardial, and collateral blood flow by pressure measurements for assessing functional stenosis severity before and after percutaneous transluminal coronary angioplasty. Circulation. 1993; 87: 1354-1367.
- Pijls NH, Van Gelder B, Van der Voort P, et al. Fractional flow reserve. A useful index to evaluate the influence of an epicardial coronary stenosis on myocardial blood flow. Circulation. 1995; 92: 3183-3193.
- Pijls NH, De Bruyne B, Peels K, et al. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med. 1996; 334: 1703-1708.
- Davies JE, Sen S, Dehbi H-M, et al. Use of the instantaneous wave-free ratio or fractional flow reserve in PCI. N Engl J Med. 2017 May 11;376(19):1824-1834. doi: 10.1056/NEJMoa1700445.
- Götberg M, Christiansen EH, Gudmundsdottir IJ, et al. Instantaneous wave-free ratio versus fractional flow reserve to guide PCI. N Engl J Med. 2017 May 11;376(19):1813-1823. doi: 10.1056/NEJMoa1616540.
- Douglas PS, Hoffmann U, Patel MR, et al; PROMISE Investigators. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015; 372: 1291-1300.
- Douglas PS, Pontone G, Hlatky MA, et al; PLATFORM Investigators. Clinical outcomes of fractional flow reserve by computed tomographic angiography-guided diagnostic strategies vs. usual care in patients with suspected coronary artery disease: the prospective longitudinal trial of FFRCT: outcome and resource impacts study. Eur Heart J. 2015 Dec 14; 36(47): 3359-3367. doi: 10.1093/eurheartj/ehv444.
Disclosure: Dr. Kern is a consultant for Abiomed, Merit Medical, Abbott Vascular, Philips Volcano, ACIST Medical, Opsens Inc., and Heartflow Inc.